Our Research Projects
Explore our innovative research topics and contributions in biomedical sciences.
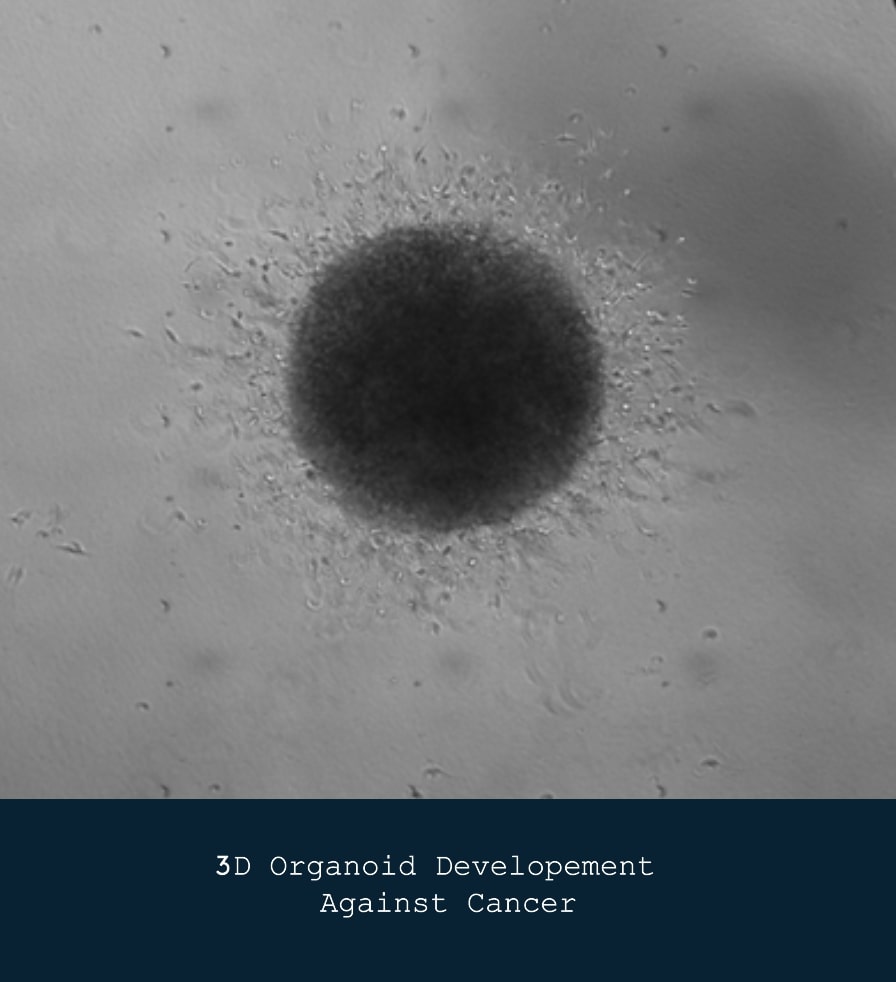
Precision Engineering Using 3D Organoid Development
Our precision engineering efforts involve the development of 3D organoid models that closely mimic the architecture and biology of human tumors. These organoids serve as powerful tools for studying tumor behavior, drug response, and therapeutic resistance. By utilizing patient-derived organoids, we aim to facilitate personalized treatment strategies and identify potential therapeutic targets that can be validated in vitro and in vivo.
These models allow us to recreate the tumor microenvironment with high fidelity, enabling us to observe cellular interactions and behaviors that are critical for understanding cancer progression.
By studying these organoids, we can uncover the mechanisms behind tumor heterogeneity and the varied responses to treatments among patients. This insight is vital for developing targeted therapies that address the unique characteristics of each tumor.
Additionally, we are exploring the integration of high-throughput screening technologies with our organoid models, which will significantly accelerate the drug discovery process.
By systematically testing a wide range of compounds, we can identify those that exhibit the most promising efficacy against specific tumor types, paving the way for clinical trials tailored to the individual patient's cancer profile.
3D Organoid platforms to study anti-invasive therapy :
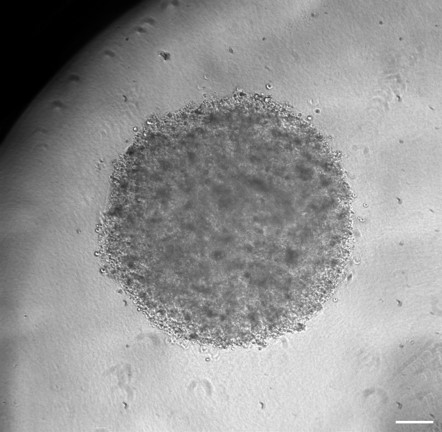
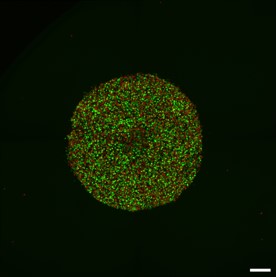
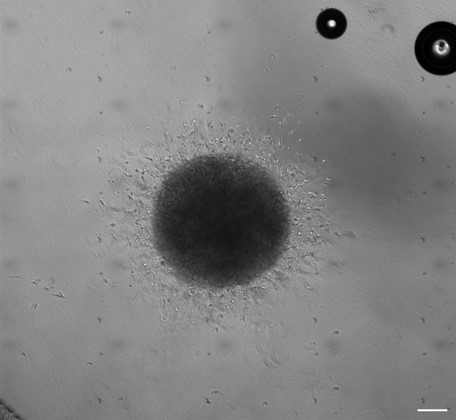
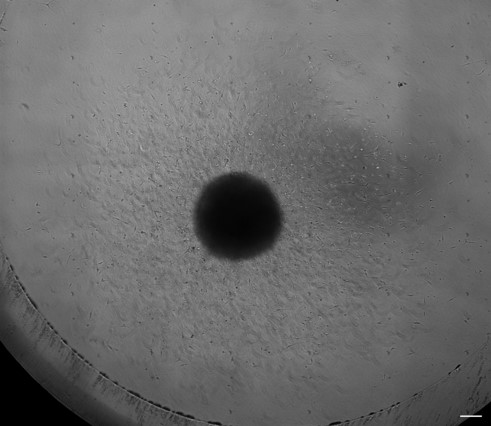
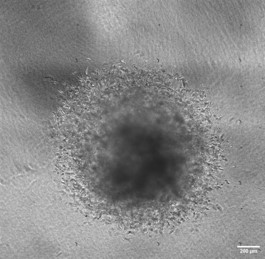
3D Organoid models representing different stages of GBM :
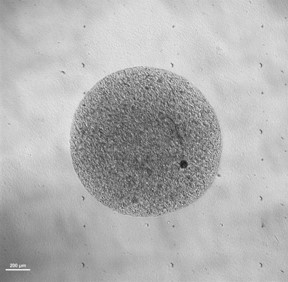
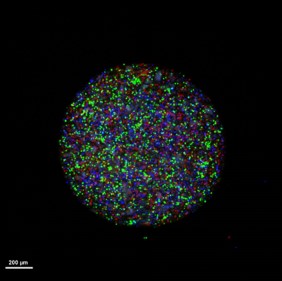
Growing organoids in distinct environment :
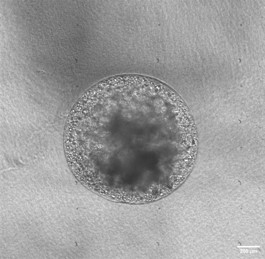
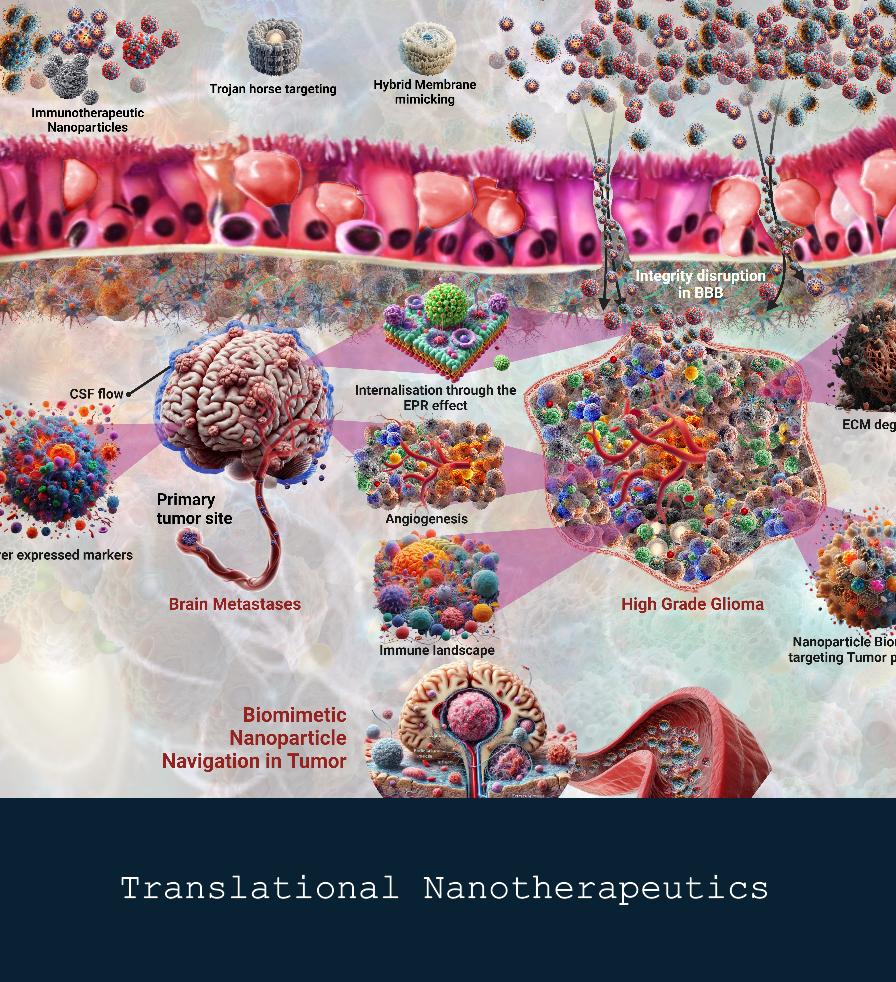
Translational Nanobiotherapeutics
Our research in translational nanotherapeutics aims to develop innovative nanoparticle-based drug delivery systems that enhance the efficacy and specificity of cancer therapies. By leveraging the unique properties of nanomaterials, we seek to improve therapeutic outcomes while minimizing systemic side effects. Our multidisciplinary approach integrates nanotechnology and molecular biology, allowing us to design tailored formulations that target tumor cells and the tumor microenvironment effectively.
Through the development of these targeted nanosystems, we are also exploring the potential of stimuli-responsive nanocarriers, which release drugs in response to specific environmental triggers, such as pH or enzyme levels within tumors. Additionally, we are investigating how nanoparticle engineering can enhance the delivery of combination therapies, optimizing the synergy between drugs and making treatment even more effective. By focusing on precision and safety, our work in nanotherapeutics aims to set new standards in personalized cancer care and treatment efficacy.
The accompanying images highlight some of the advanced techniques and outcomes of our research:
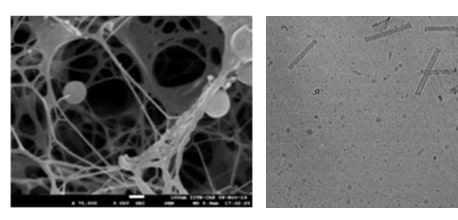
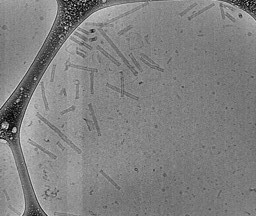
1.Nanostructure Imaging: The electron microscopy images on the left showcase the intricate nanostructures in injectable gel system developed in our lab.
These structures are engineered to provide optimal stability and drug encapsulation efficiency, ensuring controlled and targeted release. Through this patented technology, we aim to provide minimally invasive and cost affordable treatment against high-grade gliomas.
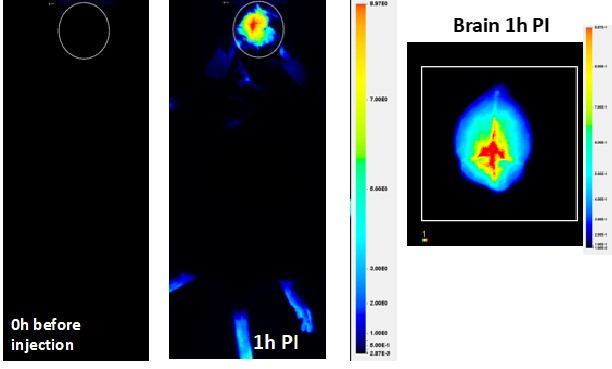
2.Blood-Brain Barrier Delivery: The middle and right panels illustrate our success in achieving drug delivery across the blood-brain barrier (BBB),
a significant challenge in treating brain cancers. Using specially formulated nanoplatforms, we've demonstrated that our system can effectively cross the BBB
and deliver therapeutic agents directly to the brain, as seen in the imaging studies one hour post-injection (1h PI). The NIR image scan indicates high levels of nanocarrier accumulation in the targeted brain tumor regions, confirming the efficacy of our delivery approach.
3.Injectable system: Our patented lipid nanovesicle in gel system, shown in the image, represents a breakthrough in localized delivery for sustained release.
By embedding these nanovesicles within a tunable gel matrix, we can enhance their regional delivery potential and minimize off-target tissue damage.
Precision Immunotherapy
We are dedicated to advancing the field of cancer immunotherapy through the exploration of novel strategies that activate and harness the immune system to combat malignancies. Our lab investigates the interplay between nanoparticles and immune cells to develop synergistic treatments that enhance antitumor responses. This includes studying immune checkpoint inhibitors and nanovaccines to create more effective and personalized therapeutic options for cancer patients.
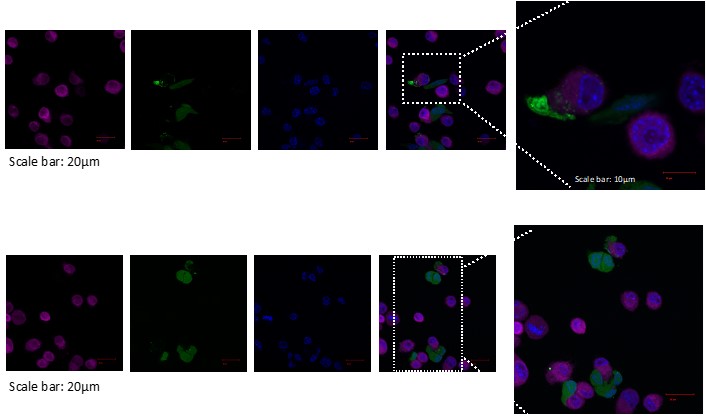
Our research focuses on understanding the mechanisms by which nanoparticles can improve the delivery and efficacy of immune-modulating agents. By leveraging the unique properties of nanoparticles,
we aim to enhance the uptake of therapeutic agents by immune cells, thereby amplifying the immune response against tumors. We are particularly interested in developing targeted nanovaccines that can
stimulate the immune system to recognize and attack cancer cells while minimizing side effects.
In addition to our work on nanoparticles, we are exploring the role of the tumor microenvironment in shaping immune responses. By characterizing the immune landscape within tumors,
we aim to identify key factors that promote or inhibit effective antitumor immunity. Our studies include the evaluation of various immune cell populations, such as T cells, dendritic cells, and macrophages,
to understand their interactions with tumor cells and the impact of therapeutic interventions
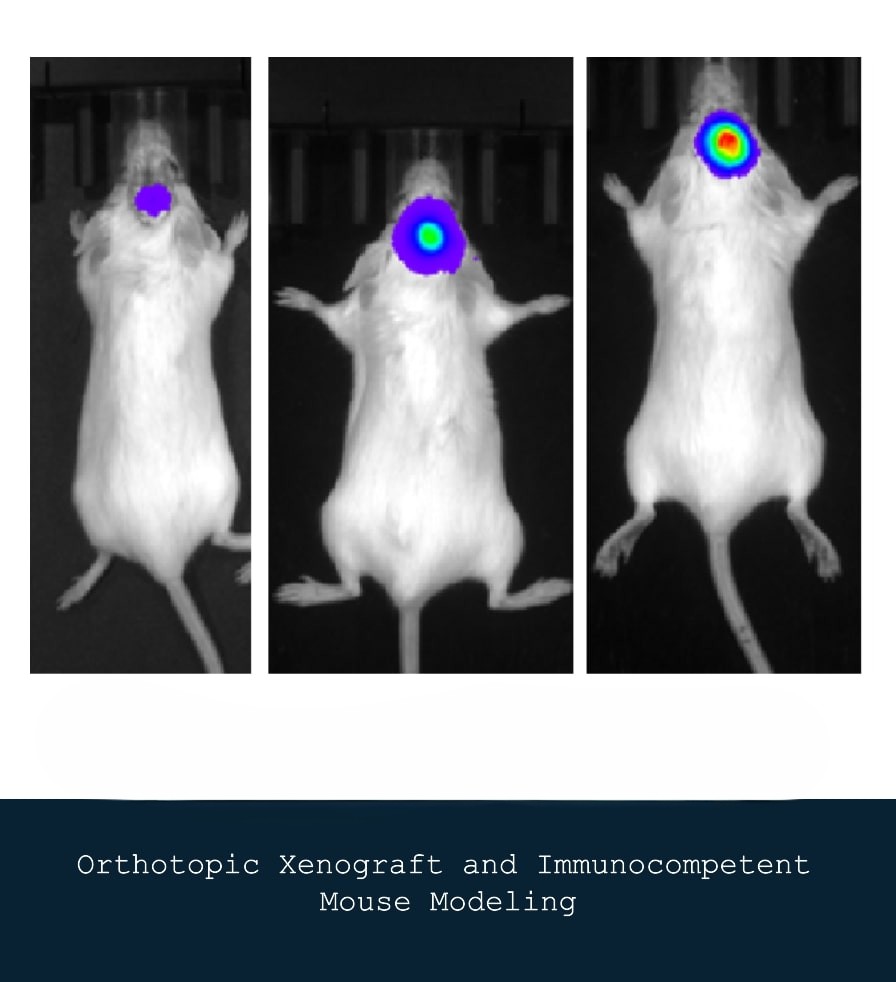
Orthotopic Patient-Derived Xenograft Mouse Modeling
We utilize orthotopic patient-derived xenograft (PDX) models and immunocompetent mouse models to study tumor growth and response to therapies in a more physiologically relevant context. These models enable us to evaluate the efficacy of novel therapeutic agents and investigate the tumor microenvironment's role in cancer progression. Our research seeks to bridge the gap between bench and bedside by providing insights that can inform clinical decision-making.
By employing PDX models, which involve implanting human tumors directly into the corresponding organ of immunocompromised mice, we can maintain the heterogeneity and complexity of the original tumor. This approach allows for a more accurate assessment of how specific therapies interact with tumor biology, leading to more personalized treatment strategies. In parallel, the use of immunocompetent mouse models helps us understand how an intact immune system interacts with tumor cells and therapeutic interventions.
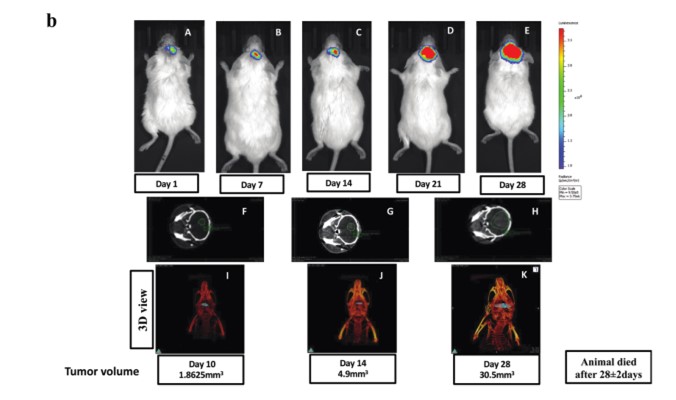
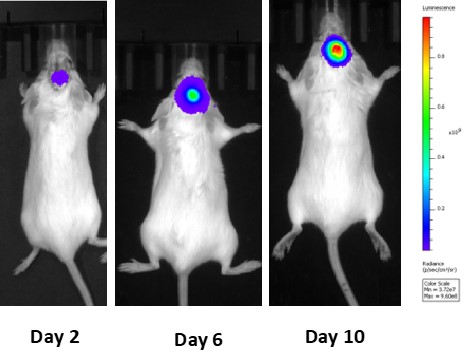
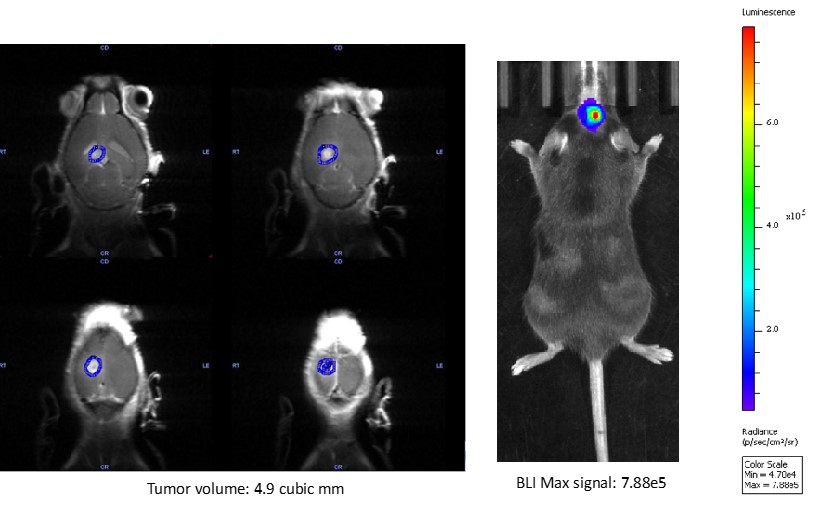
Syngeneic mouse model development for GBM :

Discovery of Biomarkers for Early Cancer Detection
Our lab is actively engaged in the discovery and validation of novel biomarkers for early cancer detection.
By employing advanced omics technologies and machine learning/quantum algorithms,
we aim to identify specific molecular signatures that can predict tumor presence and progression. Early detection is crucial for improving patient outcomes,
and our work focuses on translating these findings into clinically applicable tests that can be used for screening and diagnosis